Details about the mechanism of biofilms and their impact...
Biofilm mode of growth is an alternative lifestyle in which microbes adopt multicellular form to facilitate and enhance the lifespan in diverse environmental niche (Kostakioti, Hadjifrangiskou et al. 2013). They are surface attached communities of microbes that are ubiquitous in nature and may be mono-species or multi-species. Almost every species of microorganism possess mechanism to adhere to surfaces and with each other to form this specialized entity called biofilm. It can be developed on non-shredding surfaces in aqueous (or humid) environment. In this mode of growth bacteria cocooned themselves in self-produced extracellular matrix, that constitutes ~90% biomass(Flemming and Wingender 2010).
The concept of biofilm was established in two seminal papers published in the 1930s in the Journal of Bacteriology (JB) by Arthur Henrici (Henrici 1933) and Claude Zobell(Zobell and Allen 1935) while Bill Costerton coined the term biofilm in 1978(Costerton, Geesey et al. 1978).
Developmental stages of biofilm:
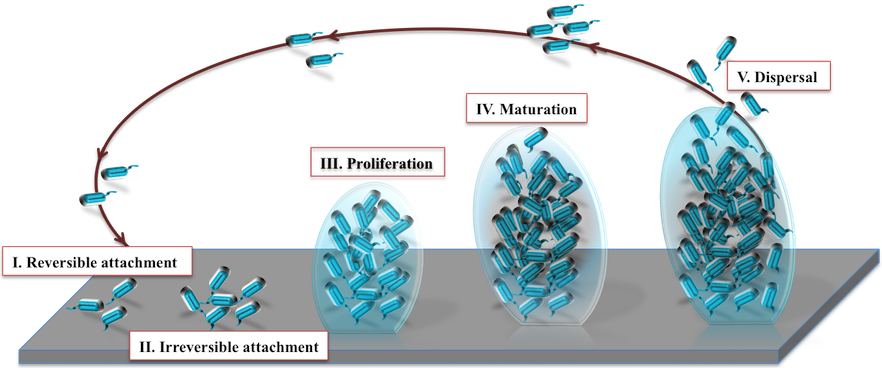
Connection between quorum sensing (QS) and biofilm termed as sociomicrobiology (Parsek and Greenberg 2005). It aids bacteria in diverse ways like survival in adverse environment, antibiotic resistance, dispersal of microbes to new sites, horizontal gene transfer, protection from immune system of host, etc.
Therefore, to cease the effect of multicellular mode lifestyle (biofilm) of microbes certain anti-biofilm agents are required. Various agents are experimentally validated: targets various stages of biofilm (adhesion, formation, maturation, dispersion), categories (chemical, phytochemical, bacterial extract, phages, peptides, etc.), mode of action (antagonizing receptor, QS signaling molecules, matrix, and so on), targeting organism (P. aeruginosa, C. albicans, E. coli, S. aureus), inhibition efficiency (0-100%), etc.
Biofilm is a specialized phenotypic transformation of bacteria from unicellular to mimic multicellular behavior for acclimatizing various environmental variations. It is considered as a consortium of microbes with monospecies or polyspecies colonization. In biofilm mode, the bacteria exhibits 10-1000 folds increase in antibiotic resistance due to their specialized features like efflux pumps, modifying enzymes, evading immune system and target mutations (Walsh 2000, Stewart and Costerton 2001, Roilides, Simitsopoulou et al. 2015). Sakhtah H et al. in 2016 demonstrated the role of multidrug efflux pump MexGHI-OpmD through a natural phenazine in the biofilm development in the opportunistic pathogen Pseudomonas aeruginosa (Sakhtah, Koyama et al. 2016). Biofilm formation increases the pathogenicity of the facultative pathogen Vibrio cholerae in the environment and during outbreaks (Fong, Syed et al. 2010). Likewise switching to biofilm form enhances the persistence of Candida albicans (Tsui, Kong et al. 2016) during mucosal to systemic infection. Biofilm matrix exoproteins have been shown to have the multivalent vaccine candidate potential against biofilm-associated chronic infections of Staphylococcus aureus as reported by Gil C et al (Gil, Solano et al. 2014). Moreover, recalcitrant nature of biofilms helps the pathogens to overcome the host innate response as well as antibiotics (Begun, Gaiani et al. 2007).
The universal distribution, varied composition, virulence and resistance towards antibiotics, make biofilm among one of the highly explored fields. The aBiofilm with all the relevant information of anti-biofilm agents with database, analyses tools and a predictor would aid the researchers to understand, explore, and design novel and effective biofilm targeting approaches. This would further intensify the research on biofilm specifically to tackle the menace of drug resistance.