| CAROTENOID INFO | |
|---|---|
| CAROTENOID NAME | Lycopene |
| STRUCTURE | 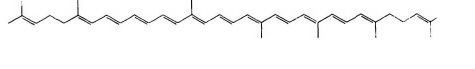 |
| OTHER NAME | all-trans-Lycopene/ trans-Lycopene |
| SYSNAME/SYNONYMS | psi,psi-carotene |
| MOLECULAR FORMULA | C40H56 |
| MOLECULAR WEIGHT | 536.87264 |
| IUPAC NAME | (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene OR _,_-carotene |
| InChI | OAIJSZIZWZSQBC-GYZMGTAESA-N |
| CANONICAL SMILES | CC(=CCCC(=CC=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC=C(C)CCC=C(C)C)C)C)C)C |
| ISOMERIC SMILES | CC(=CCC/C(=C/C=C/C(=C/C=C/C(=C/C=C/C=C(/C=C/C=C(/C=C/C=C(/CCC=C(C)C)C) C)C)/C)/C)/C)C |
| KEGG ID | C05432 |
| PUBCHEM ID | 7796 |
| CHEBI ID | CHEBI:15948 |
| UV SPECTRA | Lambda max (nm): hexane 286, 295, 425, 448, 476, 507 (Manchand et al, 1965);acetonitrile/methanol/THF (58:35:7) 296, 364, 447, 473, 504, %III/II=70 (Takaichi, 2000); n-hexane 443.0, 470.5, 502.0 (Quian et al, 2001) |
| NMR SPECTRA | 1H-NMR d(CDCl3): 5.11 (2, 2'-H), ca. 2.11 (3, 3'-H2), ca. 2.11 (4, 4'-H2), 5.95 (6, 6'-H), 6.49 (7, 7'-H), 6.25 (8, 8'-H), 6.18 (10, 10'-H), 6.64 (11, 11'-H), 6.35 (12, 12'-H), 6.25 (14, 14'-H), 6.62 (15, 15'-H), 1.688, 1.612 (1, 1'-gem-Me), 1.818 (5, 5'-Me), 1.968 (9, 9', 13, 13'-Me) (Hengartner et al, 1992) 13C-NMR d(CDCl3): 131.64 (1, 1'), 124.12 (2, 2'), 26.83 (3, 3'), 40.30 (4, 4'), 139.30 (5, 5'), 125.94 (6, 6'), 124.87 (7, 7'), 135.54 (8, 8'), 136.15 (9, 9'), 131.64 (10, 10'), 125.21 (11, 11'), 137.46 (12, 12'), 136.54 (13, 13'), 132.71 (14, 14'), 130.17 (15, 15'), 25.66, 17.70 (1, 1'-gem-Me), 16.97 (5, 5'-Me), 12.90 (9, 9'-Me), 12.81 (13, 13'-Me) (Hengartner et al, 1992) (CDCl3) (Qian et al, 2001) 1H- and 13C-NMR in CDCl3 (Qian et al, 2002) |
| MASS SPECTRA | m/z: 536 (M, 22%), 467 (M-69), 444 (M-92), 430 (M-106), 378 (M-158), 361 (M-106-69)(Enzell et al, 1969; Schwieter et al, 1969) ; FD-MS m/z: 536 (Kuwabara et al,1999/ Wang et al,2012) |
| HPLC CHROMATOGRAM | RF-TLC on 0.25 mm RP-18 layers (Merck, Art. 15423) using several ratio of light petroleum (bp 40-60C)-acetonitrile-methanol ex: 1:6:3 Rf=0.16, 3:1:6 Rf=0.27 ( Isaksen & Francis,1986); HPLC (column: Nucleosil-300-5 0.4times50 cm, eluent: hexane-N-ethyldiisopropylamine, 2000:1, flow: 0.6 ml/min, detect : 469 nm) tR = ca. 27 min (all-E isomer) (Hengartner, 1992) ; HPLC (column; Novapak C18 (Waters) 8 X 100 mm: eluelnt; acetonitrile/methanol/THF 58:35:7: flow 2.0 ml/min) Rt=11.4 min (Takaichi,2000) |
| OTHER SPECTRA | IR spectra :KBr disc or pellet (Watanabe et al, 2000);Fluoresence (Akimoto et al, 2000); Raman spectrum in n-hexane (Qian et al, 2001) |
| REFERENCES |
1. Akimoto S, Yamazaki I, Takaichi S, Mimuro M: Excitation relaxation dynamics of linear carotenoids. J. Lumin. 2000, 87:797-799.
2.Enzell CR, Francis GW, Liaaen-Jensen S, Schultes RE, Lindberg AA, Jansen G, Lamm B, Samuelsson B: Mass Spectrometric Studies of Carotenoids. 2. A Survey of Fragmentation Reactions. Acta Chem. Scand. 1969, 23:727-750.(PMID: 5806308) 3. Hengartner U, Bernhard K, Meyer K, Englert G, Glinz E: Synthesis, isolation, and NMR-spectroscopic characterization of fourteen (Z)-isomers of lycopene and of some acetylenic didehydro- and tetradehydrolycopenes. Helv Chim Acta, 1992, 75: 1848-1865. 4. Isaksen M, Francis GW: Reversed-phase thin-layer chromatography of carotenoids. J. Chromatogr.1986 , 355:358-362. 5.Kuwabara T, Hasegawa M, Kawano M, Takaichi S: Characterization of violaxanthin de-epoxidase purified in the presence of Tween 20: effects of dithiothreitol and pepstatin A. Plant Cell Physiol. 1999, 40:1119-26.(PMID: 10635115) 6.Manchand P.S, RŸegg R, Schwieter U, Siddons P.T, Weedon B.C.L: Carotenoid and Related Compounds. Part XI. Syntheses of delta-Carotene and epsilon-Carotene. J. Chem. Soc., Pert II. 1965, 2019 -2026 7. Qian P, Mizoguchi T, Fujii R, Hara K: Conformation analysis of carotenoids in the purple bacterium Rhodobium marinum based on NMR spectroscopy and AM1 calculation. J. Chem. Inf. Comput. Sci. 2002, 42:1311-9.(PMID- 12444727) 8.Qian P, Saiki K, Mizoguchi T, Hara K, Sashima T, Fujii R, Koyama Y: Time-dependent changes in the carotenoid composition and preferential binding of spirilloxanthin to the reaction center and anhydrorhodovibrin to the LH1 antenna complex in Rhodobium marinum. Photochem. Photobiol. 2001, 74:444-52.(PMID- 11594059) 9.Schwieter U, Englert G, Rigassi N, Vetter W: Physical organic methods in carotenoid research. Pure Appl. Chem. 1969, 20:365-420.(PMID: 5377333) 10.Takaichi S: Characterization of carotenes in a combination of a C(18) HPLC column with isocratic elution and absorption spectra with a photodiode-array detector. Photosynth. Res. 2000, 65:93-9. (PMID:16228474) 11.Wang G-S, Grammel H, Abou-Aisha K, SŠgesser R, Ghosh R: High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78:7205-15.(PMID: 22865070) 12.Watanabe K, Yasugi E, Oshima M: "How to search the glycolipid data in LIPIDBANK for Web: the newly developed lipid database" Japan Trend Glycosci. and Glycotechnol. 2000, 12, 175-184.(PMID:12058481) |
| CAROTENOID INFO | |